What are the types of intermolecular forces?
2 min read•december 21, 2021
AP Chemistry 🧪
269 resourcesSee Units
There are many types of intermolecular forces (IMFs).
- Learn more by watching this informative 🎥 video reviewing all the intermolecular forces
The first of these is the set of Vander Waals forces, which do not involve ions. Within this set, there are three main types of forces:
London Dispersion Forces
- These forces do not involve polar molecules.
- AKA Induced dipole - induced dipole forces
- All molecules experience LDFs (since all have an induced dipole)
- Examples: Br₂, I₂
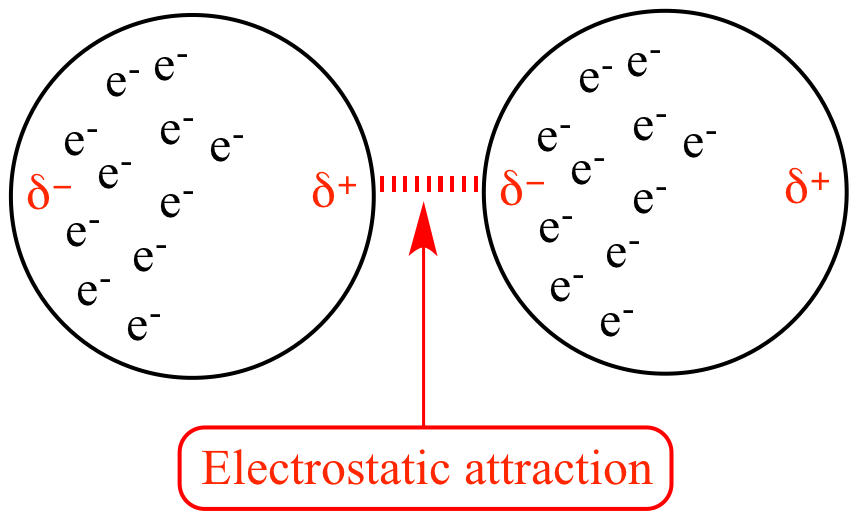
Note that in the image above, these are induced dipoles - basically electrons being in random places creates incredibly weak dipoles (This is why LDFs are so weak!)
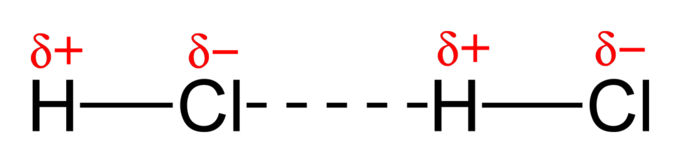
Dipole-Dipole Forces
- These forces do involve polar molecules.
- Examples: CH₃Cl, PCl₃
Hydrogen Bonding
- These forces involve hydrogen and nitrogen, oxygen, or fluorine. You can remember this through the mnemonic device NOF.
- Examples: H₂0, NH₃, HF
- Fun fact: Hydrogen bonding is why water has so many unique properties!
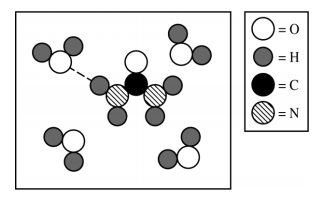
Source: 2019 AP Exam Scoring Guides
Ion-Dipole Forces
- These forces involve both polar molecules and ions, and are typically salts in water.
- Examples: NaCl in H₂0, NBr in H₂0
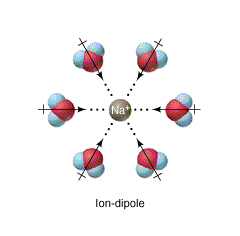
It is important to note that ionic bonding is absent from this list of IMFs. Technically speaking, ionic bonds are not intermolecular forces due to the lack of covalent bonds.
- As strength of the bond/force increases, so do melting and boiling point.
- In order from weakest to strongest forces:
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.