Dalia Savy
Jeremy Kiggundu
Dalia Savy
Jeremy Kiggundu
AP Chemistry 🧪
269 resourcesSee Units
If you recall from the last section, matter can be categorized according to state or composition. The three states of matter will be elaborated upon in unit three of this AP Chemistry course, but we will delve deeper into categorizing matter by composition in this guide.
Remember that the first division in the classification of matter by composition is between a pure substance and a mixture. A pure substance is composed of a single type of atom or molecule, whereas a mixture is a composition of two or more elements or compounds.
What are Mixtures?
As discussed, mixtures are materials made up of two or more substances that are physically combined.
Formula Units: Pure Substances and Mixtures
To better clarify the difference between pure substances and mixtures, let's discuss them in terms of formula units. As a review, a formula unit represents the lowest whole number ratio of atoms that can be used to describe a compound.
While pure substances contain atoms or formula units of a single type, mixtures contain atoms or formula units of two or more types (and their proportions vary).
Different Types of Mixtures
Just like there are different types of pure substances, there are different types of mixtures!
💭 Remember that a pure substance can be either an element or a compound. Both are composed of a single type of atom or molecule, but compounds can be chemically decomposed into smaller parts (elements).
Mixtures are separated into two categories: homogeneous and heterogeneous.
- Homogeneous mixtures are composed of two or more substances but are uniform in composition. In other words, if you take a look at a homogeneous mixture, you will not be able to see the parts it is made up of.
- 🌊 Salt water is made up of different compounds (water + salt) but you can't see ALL of the elements; it all looks like one mixture of just water.
- 💨 Air is a homogeneous mixture made up of gases. So many different gases make up air, but it is impossible to see its composition.
- An important characteristic of homogeneous mixtures is that they are difficult to separate. Imagine trying to take a bottle of soda and separating it, how would that even work?
- Heterogeneous mixtures are visibly composed of more than one element or compound. Their composition is not uniform, so the proportions of different compounds can vary!
- 🍨 Rocky road ice cream is a prime example of a heterogeneous mixture. You could see each of the "compounds" (chocolate ice cream, nuts, and marshmallows).
- 🥗 Salad is another heterogeneous mixture since you can see all of the ingredients you physically combined in a bowl.
- Unlike homogenous mixtures, heterogeneous mixtures are often separable. Their components are physically distinct from one another, so it is pretty easy to disassemble them. Think about taking a salad apart!
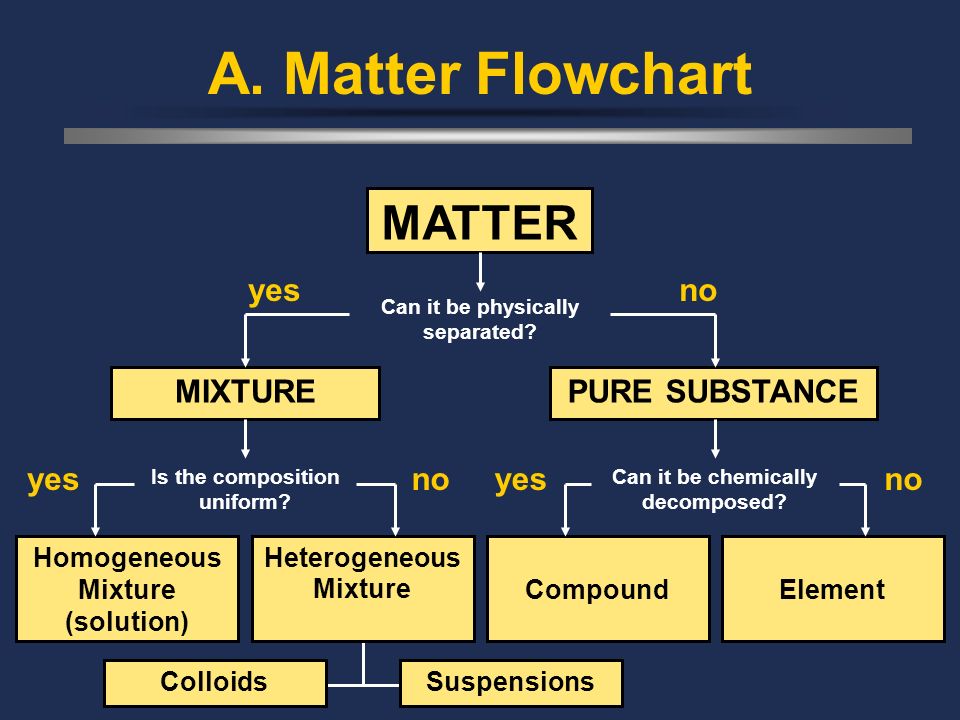
This describes the categorization of matter, taking what we are discussing in this guide and combining it with what we discussed in the last! This only includes the separation of matter based on composition, not state.
Separating Mixtures
Chemists often want to separate a mixture into its components in chemical experiments. There are several ways to separate mixtures, but the most common ways in chemistry are distillation and filtration. These techniques use different physical or chemical properties of the components in a mixture in order to separate them.
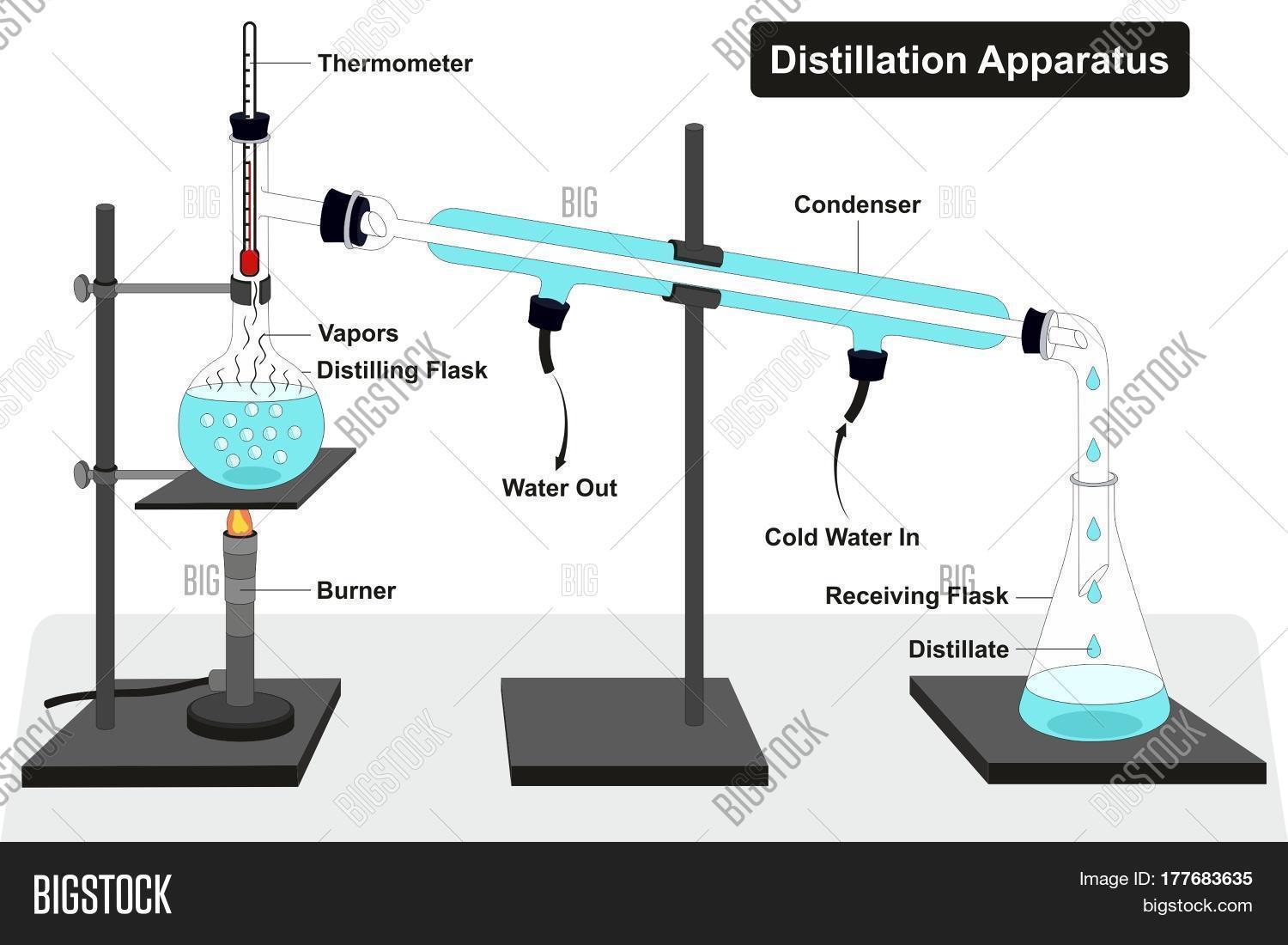
Distillation
Distillation separates components in liquid mixtures by evaporating the most volatile substance first, usually the substance with the lowest boiling temperature. The most important thing to know is that it separates based on differences in boiling points.
Think about a mixture of water and alcohol. Since the boiling point of alcohol is lower than that of water, the alcohol is going to evaporate first. Therefore, the vapor collected will contain more alcohol than water.
Filtration
Filtration separates mixtures by using a mesh or filter to separate solids from liquids. However, this only works with heterogeneous mixtures.

Imagine you are filtering a solution of salt, water, and sand. Can you guess what will get caught up in the filter paper? Only the sand will. This is because the salt will dissolve in the water since it is soluble.
Only insoluble substances will be filtered with filtration.
The filtrate would then be both salt and water, or salt water. Therefore, filtration isn't effective if you want to fully separate all of these elements. After filtering the sand out, you would have to evaporate the water out of the salt water solution to separate the salt from the water.
Thin-Layer Chromatography (TLC)
Chromatography is a method used to identify and compare mixtures based on their attraction to solids and liquids or differences in polarity (polarity is further explained in future units).
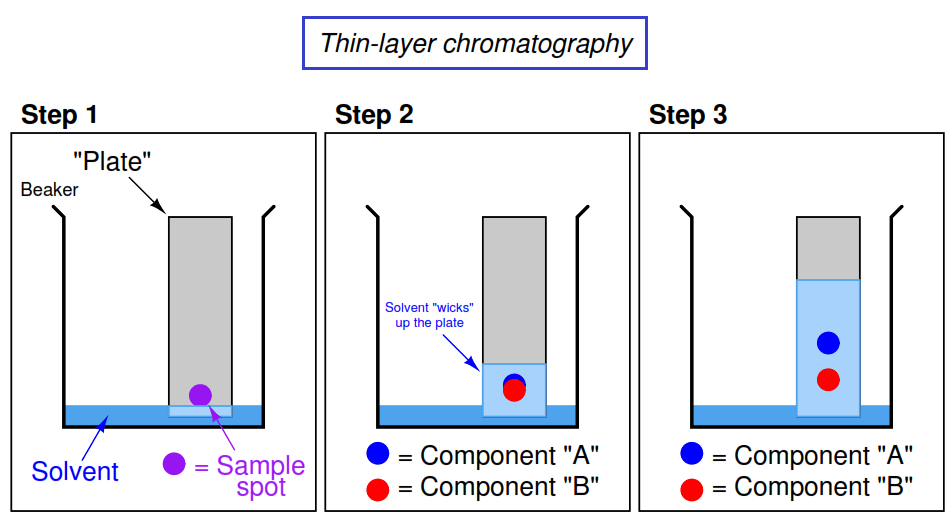
Image Courtesy of Instrumentation Tools
In the first step, someone drew a dot with a marker. Most of the ink in markers are a mixture of a number of substances and thin-layer chromatography can separate these substances.
In step two, the solvent climbed up the filter paper📄 and began to separate the sample spot by its substances.
A quick rundown of polarity to explain TLC:
Polarity is one way to compare solvents or substances. A substance may be polar or nonpolar.
You may hear the phrase, "Like dissolves like" a lot. This means polar substances dissolve in polar solvents and nonpolar substances dissolve in nonpolar solvents.
Do you know how oil and water never mix? Water is a polar substance and oil is a nonpolar substance. Since they aren't the same polarity, they never mix.
Most TLC plates are made up of polar silica, which you can think of as a very small powder. This silica is considered the stationary phase, while the solvent in the TLC chamber is considered the mobile phase. A general rule of thumb is that polar compounds are more strongly attracted to the stationary phase, and will move less, while nonpolar compounds are more readily eluted with the solvent.
☝️Let's refer back to the image above. If the solvent is nonpolar, could you guess which component is more nonpolar? If Compound A traveled farther up the TLC plate, that means it was more readily eluted with the solvent. With this being said, Compound A is less polar than Compound B.
AP NOTE: There are other types of chromatography as well but this one is the most commonly tested on the AP Chemistry Exam.
AP Practice Question - 2018 #2
This question is adapted from the 2018 AP Chemistry Exam posted on the College Board website.
There are questions like this one on several past AP exams. This is only part a, but it's good to recognize what they are asking here. They basically want you to draw the reactant mixture given the information you are provided with.
The two things you want to note in this question are:
- The types of compounds that should be drawn in the reactant mixture
- How much of each element is present in the product mixture
Step 1) This question introduces the reaction and tells us that the two reactants are NO and O2.
Step 2) If you count how many atoms of each element are present, you would get 8 N atoms and 12 O atoms.
Since you have fewer Nitrogen atoms, it'd be good to draw the NO reactant first. Draw as many as you can until you run out of nitrogen atoms. This would get us to have 8 NO molecules. Now, we have 4 O atoms remaining. Since each O2 compound contains two atoms of oxygen, we could only draw 2 O2 molecules with the remaining oxygen.
A sample response looks like the following:
Make sure you use the key they gave you when drawing the molecules! You should always look out for requirements and directions on how to draw diagrams. The orientation at which you draw the molecules doesn't matter, as long as you draw them correctly.
AP Practice Question - 2017 #4
This question is adapted from the 2017 AP Chemistry Exam posted on the College Board website.
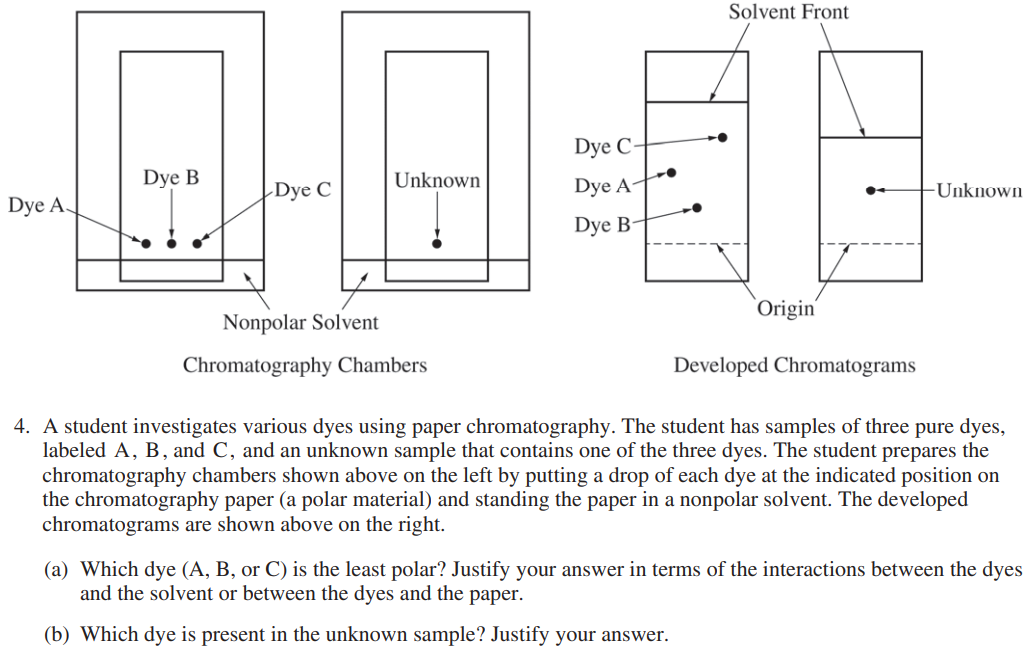
What You Know
This is an AP free-response question testing your knowledge about thin layer chromatography! In this question, they are giving you several pieces of information:
- You have two TLC plates developing: one with labeled and known dots, and one with an unknown dot.
- You are developing your TLC plates in a nonpolar substance.
- You are told where the dots began, so you can compare them with regard to the distance they traveled from the origin.
Breaking down part a:
Part (a) is asking you which of the known dyes (A, B, or C) is the least polar and to justify your response. Since they explicitly asked, your justification must involve the interactions between the dyes and the solvent or the dyes and the paper.
You should automatically be able to eliminate A as the least polar dye, since it travels a distance between C and B. You can see that dye C travels the farthest in a nonpolar solvent, while dye B has traveled the least. Remember "like dissolves like," so the least polar dye, or the most nonpolar dye, will travel the farthest in a nonpolar solvent!
Therefore, your answer to part a should be similar to one of the following:
"Dye C is the least polar dye because it moved the farthest on the TLC plate. This is because nonpolar dyes are more attracted to the nonpolar solvent."
"Dye C is the least polar dye because it moved the farthest on the TLC plate. This is because nonpolar dyes are less strongly attracted by the polar TLC plates."
Both of these responses would earn you the full two points on this part of #4.
Breaking down part b:
Part (b) asks you to figure out which dye is present in the unknown sample and justify. This question can easily check many students if they aren't paying close attention to the difference in the TLC plates.
The solvent in the developed TLC plates with the dyes has traveled much farther than the one with the unknown. This is important to note because in order to accurately tell which dye is in the unknown, the solvent fronts must be in the same location. Therefore, we have to use proportions to answer this question.
The unknown dye seems to have traveled half the distance that the solvent did, so we have to find which dye did the same on the other TLC plate. It looks like dye A followed the same behavior, so it must be in the unknown.
According to the scoring guidelines, either of the following responses would earn you a full two points:
"Dye A is present in the unknown sample. The unknown sample moves to a position that is midway between the origin and the solvent front, and so does dye A"
Dye A has a retention factor (Rf) that is close to 0.50 on the chromatogram with the three dyes, and the unknown also has a retention factor close to 0.50."
We haven't discussed Rf yet, but it is basically a measure of the distance traveled by the dye relative to the distance traveled by the solvent. This number is always going to be between 0 to 1.
Rf = (distance traveled by the component) / (distance traveled by the solvent)
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.