6.3 Kinetic Energy, Heat Transfer, and Thermal Equilibrium
2 min read•january 29, 2023
Dalia Savy
A
Anika P
Dalia Savy
A
Anika P
AP Chemistry 🧪
269 resourcesSee Units
Molecular Collisions
Temperature reflects the average kinetic energy, or random motion, of particles. Kinetic energy is speed, so as temperature increases, molecules collide with the walls of the container faster.
The Collision Theory
Collision theory is based on the following postulates:
- The rate of a reaction is proportional to the rate of the collisions 💨
- Orientation matters for the reactant molecules colliding ➡️⬇️
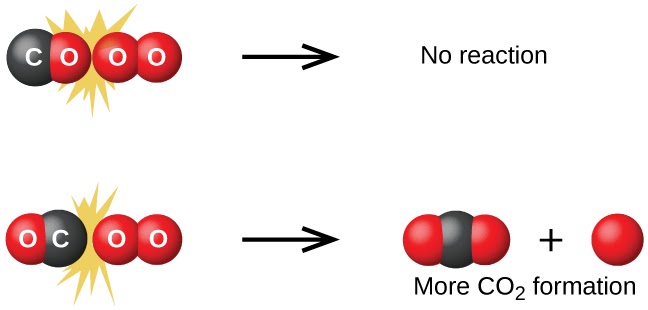
Image Courtesy of Chemistry BC Textbook
To understand how collision theory works, it's useful to think of an analogy to break down the molecular processes. Let's say you're making lemonade. To make lemonade, you need to mix lemon juice, sugar, and water together. But just having the ingredients in the same place isn't enough to make lemonade - they need to interact with each other in just the right way.
Collision theory is like the "rules" for how ingredients can mix together to make a reaction happen. It says that for two things to react and form a new substance, they need to bump into each other with enough energy, in the right orientation, and often enough. Think of it like two billiard balls - if they don't hit each other hard enough, or at just the right angle, they won't react.
And just like how making lemonade faster would require you to mix the ingredients more quickly, the theory also explains how increasing the temperature or concentration of reactants can make the reaction happen faster by increasing the number and energy of collisions.
Successful collisions would therefore collide with proper orientation to break bonds and have enough energy to overcome the reaction’s activation energy.
Just remember, proper orientation + enough energy = successful collision.
If we look at this in terms of heat transfer, higher temperatures mean a higher number of collisions because the reactants are moving quickly. Heat conduction involves this high temperature state going to a low temperature state, where collisions are not as frequent due to the decrease in temperature.
Transfer of Heat Between Objects
As we see in real life, heat travels from a hot object to a cold object until the two are in thermodynamic equilibrium (thermal equilibrium). Take for example, putting a pan🍳 up against hot grates on a stove that are heated up by a fire. The heat travels from the grates to the pan until the two are the same temperature.
Basically, the heat goes from the source (hot item) to the sink (cold item), until thermal equilibrium is reached. At thermal equilibrium, molecules are moving at the same speed since the temperatures are the same.
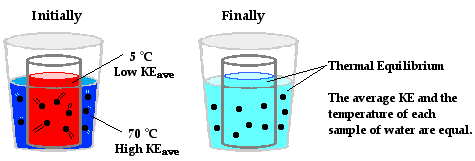
Image Courtesy of ThePhysicsClassroom
The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics is stated as "if a is in thermal equil. with b and b is in thermal equil. with c, then a is in thermal equilibrium with c" where a, b, and c are bodies.
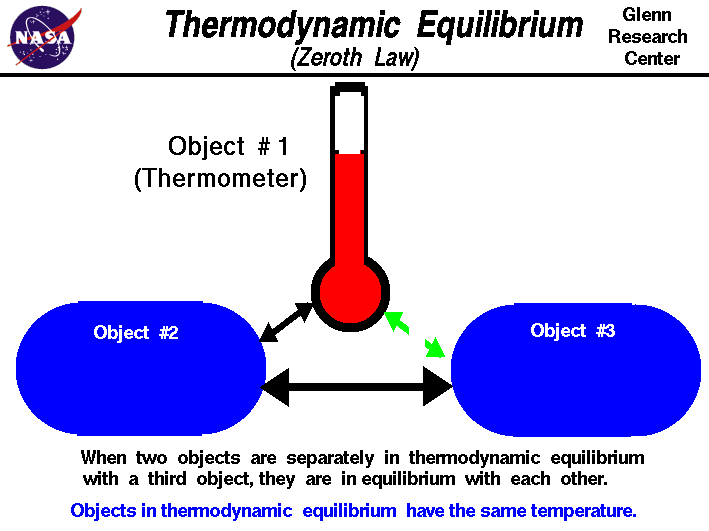
Image Courtesy of NASA
Browse Study Guides By Unit
⚛️Unit 1 – Atomic Structure & Properties
🤓Unit 2 – Molecular & Ionic Bonding
🌀Unit 3 – Intermolecular Forces & Properties
🧪Unit 4 – Chemical Reactions
👟Unit 5 – Kinetics
🔥Unit 6 – Thermodynamics
⚖️Unit 7 – Equilibrium
🍊Unit 8 – Acids & Bases
🔋Unit 9 – Applications of Thermodynamics
✏️Frequently Asked Questions
✍️Free Response Questions
🧐Multiple Choice Questions
📆Big Reviews: Finals & Exam Prep

© 2023 Fiveable Inc. All rights reserved.